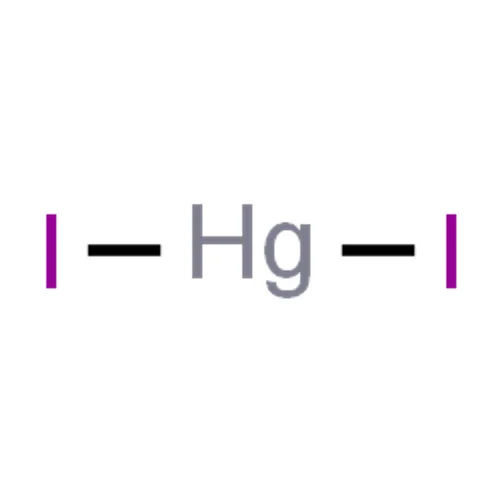
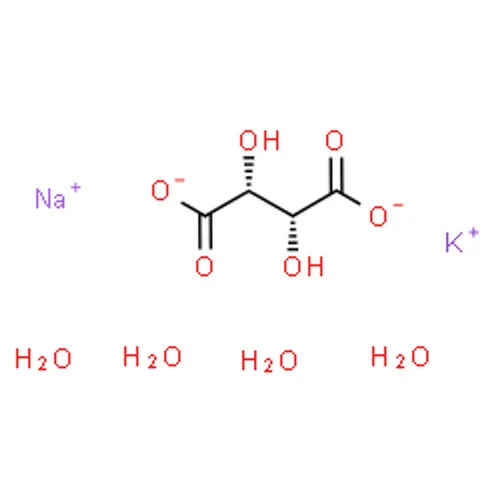
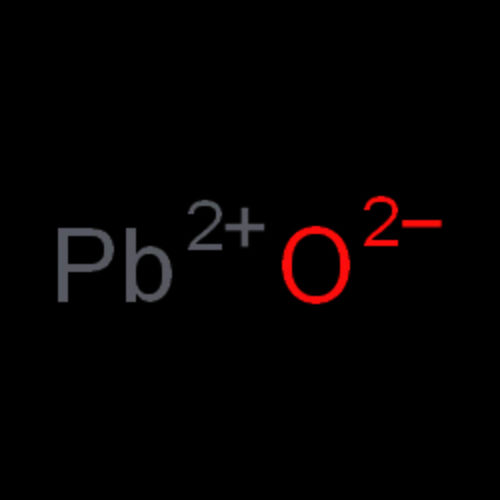Showroom
Lead Monoxide Litharge is being manufactured in our company and is widely known for its numerous attributes such as high performance efficiency, easy installation, accurate composition, smooth texture and reliability.
Lead Acetate Trihydrate find its application in various food processing, chemicals, pharmaceuticals, paint and other industries for various purposes. It is highly in demand for its accurate compositions, high performance efficiency and reliability.
Lead Tetra Acetate is extensively used in pharmaceuticals, chemical, metallurgical and textile industries and comes in different sizes of packaging that are leakage proof as well as tear resistant.
Lead Nitrate has a longer working life, accurate composition, precise pH level high performance efficiency. It is widely used in various industries such as food processing, textiles, chemical, pharmaceutical and more.
Lead Peroxide is widely used for producing diverse kinds of paints and pigments due to its high performance efficiency, accurate composition, sophisticated packaging, reliability, precise pH level and many more.
Mercuric Chloride is highly appreciated by the customers for its enormous attributes such easy usage, excellent packaging, high performance efficiency, low maintenance, long lasting service, reliability and many more.
Mercuric Oxide is ideal for making paint, pigment, fertilizers, dye and many other products. It comes in sophisticated packaging that is leakage resistant and several quantities according to the requirements and demands of the patrons.
Potassium Sodium Tartrate is formulated by using best grade chemical components and advanced methods which makes it highly durable, easy to use, smooth in texture, accurate in composition, reliable and so on.
Disodium Tartrate is widely used for producing ceramic, paint, pigment, dye and other products. It comes in a sophisticated packaging that is leakage proof and tear resistant in order to avoid any kind of corrosion.
A chemical compound called mercuric sulphate is available in the form of white crystalline powder. Its composition includes sulphur, mercury and oxygen. Its main purpose is to be used in manufacturing of other chemicals in different industrial sectors. Be careful while using and handling it as it is corrosive and highly toxic in nature.
Ammoniated mercuric chloride is a chemical compound that finds use in analytical chemistry, medicine, and photography. The white crystalline powder is composed of mercury (II) chloride and ammonium chloride. It is supplied in air-tight containers.
"We have the capability, expertise, and infrastructure to support Contract Manufacturing for other Lead & Mercury Products too"
 |
L. S. CHEMICALS AND PHARMACEUTICALS
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |